曲线型微纳拓扑结构的制备及细胞行为调控  下载: 996次【增强内容出版】
下载: 996次【增强内容出版】
The fields of tissue engineering and regenerative medicine have seen significant advancements, prompting extensive research into interactions between biomaterials and cells. Factors, such as surface chemical composition, stiffness, and topography, can influence cell adhesion, proliferation, migration, and differentiation. Advanced micro-nanofabrication technologies have made it possible to study cell-responsive topographical structures. Many cell types exhibit contact guidance behavior on groove structures, which in turn affects cell function. However, in vivo, normal tissues often exhibit curved and wavy extracellular matrix (ECM) structures, which differ significantly from previously fabricated regular topographies. Hence, in this study, maskless optical projection lithography (MOPL) was utilized to fabricate broken lines and wavy structures with varying curvatures. Groove structures were also created as controls. We examined cell migration behavior and morphological changes on the fabricated structures, proposing a cell migration mechanism specific to curved structures. This research aims to enhance our understanding of cell-surface topography interactions and provide valuable insights for designing implant materials.
A series of micro-nano topography structures were successfully created using maskless optical projection lithography. The surface morphologies of these structures were characterized via scanning electron microscopy (SEM) and atomic force microscopy (AFM). To promote cell adhesion, the fabricated structures were treated with O2 plasma and coated with poly-D-lysine (PDL). Cells were then cultured on the topography structures, and their migration behavior was monitored using a live cell station. After cell culture on the topography structures for 24 h, the cells were fixed and labeled with DAPI and phalloidin fluorescent probes for nuclei and actin, respectively. Quantitative analysis of cell roundness, cell perimeter, and cell spreading area was conducted to determine changes in cell morphology. The fabricated topography structures were found to induce cell patterning, and their biocompatibility was confirmed via cell co-culture experiments.
We successfully fabricated grooves, broken lines, and wavy structures using the MOPL technique. SEM images revealed the integrity and uniformity of the topography structures, while AFM images displayed 3D morphology of these structures. According to the AFM cross-sectional profiles, ridge heights within individual topography structures are consistent. Moreover, we subjected the surfaces of these structures to the same chemical treatment, thus isolating the impact of structural topography on cell behavior. Cell migration tracking experiments demonstrated that cells form straight, broken, and curved migration paths in accordance with the underlying topography. This finding suggests that topography structures significantly influence cell migration. Interestingly, cells on wavy structures with high curvature alter their original migration direction from along the ridge to along the curve. High curvature wavy structures prompt cell migration in the curved direction. Confocal fluorescence imaging revealed cell morphology on various topography structures. We observed that cells elongate and migrate along ridges in line regions, whereas they display reduced elongation and adopt curved morphologies in corner regions. In the corner regions of broken-line structures, cells exhibit right-angled morphologies. Actin stress fibers in cells align with the curved direction in high-curvature wavy structure corners whereas these fibers align with ridge direction in wavy structure line regions. We propose that this alignment represents an intrinsic mechanism driving cell migration in curved directions. Furthermore, we cultured cells on annular and hexagonal structures, resulting in cell patterns influenced by the induction effect of curved structures on cell migration. Live/dead fluorescence staining revealed that cells grown with the Mito-Tracker Green channel thrive, with almost no dead cells detected in PI channels. This finding suggests that the fabricated topography structures exhibit excellent biocompatibility.
We fabricated custom-designed topography structures using the MOPL technique, which presents a flexible and efficient method for creating micro/nanostructures. The cells exhibit migration trajectories that strictly adhere to the topography of grooves, broken lines, and wavy structures. In line regions, cells adopt an elongated morphology, while they assume a polygonal shape and increased roundness in corner regions of the topography structures. We posit that the high curvature of wavy structures generates an induction effect on cells, causing cytoskeletal rearrangement and cell migration along the curved direction. This observation suggests that topography plays a role in directing cell migration. By designing patterned structures based on cells' responsiveness to wavy structures, cell patterning can be realized. This study demonstrates that topography can significantly alter cell morphology and migration, enhancing our understanding of cell response mechanisms to topography. Additionally, our findings serve as a reference for designing implant materials.
1 引言
细胞微环境作为细胞生命活动的场所,由细胞外基质(ECM)、同型或异型细胞及生长因子等生物因子组成[1-3]。细胞能够感知微环境中的物理化学信号并将其转导为生物信号,从而改变形态和功能[4-6]。近年来,研究人员通过在体外模拟细胞微环境对细胞的黏附、增殖、迁移和分化等行为进行了大量的研究。如,通过在特定形貌的基底上涂覆细胞源基质,可以增强细胞骨架收缩并诱导骨髓间充质干细胞的成骨分化[7]。将精氨酸-甘氨酸-天冬氨酸(RGD)肽序列接枝到聚二甲基硅氧烷(PDMS)表面,可促进人成纤维细胞的黏附、增殖和胶原分泌[8]。除化学组分外,ECM的物理性质也是影响细胞行为的重要因素。Engler等[9]在体外构筑了不同硬度的水凝胶基底,研究了间充质干细胞的分化行为,发现在与大脑基质硬度相似的基底上,细胞倾向于向神经细胞分化,而在与骨骼硬度相似的基底上细胞倾向于向成骨细胞分化。
随着微纳加工技术的发展,ECM表面拓扑结构与细胞的相互作用引起了越来越多研究者们的兴趣[10-12]。沟槽结构作为最典型的拓扑结构已经被证明可以使多种细胞产生接触导向行为,且细胞的迁移速度与平面基底相比得到了明显的提升[13-17]。细胞在柱状结构表面通常具有较小的黏附强度和铺展面积[18-20]。然而,规则形状的沟槽结构和凸起结构并不能充分反映细胞与微环境几何拓扑结构的相互作用。体内正常组织的ECM常表现为弯曲或波浪形拓扑结构[21-24]。因此,在体外构筑多样化拓扑结构有助于进一步理解细胞对ECM表面形貌的响应机制。
传统紫外曝光[25]、电子束光刻[26]、聚焦离子束光刻[27-28]、纳米压印[29]和静电纺丝[30]等微纳加工技术已被广泛用于微纳拓扑结构的制备,但仍然难以满足大面积、高精度和高灵活性的微纳结构的制备需求。飞秒激光直写虽然可以灵活地实现任意结构的加工,但在制备大面积拓扑结构时效率较低[31-35]。飞秒激光无掩模光学投影光刻(MOPL)技术不需要实体掩模版、加工工艺简单且成本低[36-41],可同时进行多像素点加工,在制备大面积结构时具备高效性[42-43]。在前期工作中,利用水凝胶材料和MOPL技术制备了不同形状的基底结构,发现结构形貌对细胞行为起到了主要的调控作用[41]。因此,MOPL技术有望实现图案化拓扑结构的快速制备。
本文利用MOPL技术制备了一系列曲线型微纳拓扑结构,并将其应用于细胞行为调控。通过活细胞工作站对细胞在拓扑结构上的动态迁移行为进行延时成像观察。进一步地,利用荧光染色对拓扑结构上的细胞形态进行可视化观察,并对细胞圆度、细胞周长和细胞铺展面积进行了量化分析,提出了细胞在曲线型拓扑结构上的迁移机制。并且,我们制备了圆环形和六角形图案化拓扑结构,细胞能够严格按照图案化结构形貌进行定向迁移,从而实现细胞图案化。细胞共培养实验验证了所制备的拓扑结构基底具备良好的生物相容性。该研究揭示了曲线型微纳拓扑结构对细胞迁移和细胞形态的影响规律,加深了拓扑结构与细胞相互作用机制的理解,为细胞图案化以及体外植入材料的设计提供了科学依据。
2 实验部分
2.1 实验试剂及仪器
本实验中所用的试剂包括:正性光刻胶AZ P4620;显影液AZ 400K;1,2-丙二醇单甲醚醋酸酯(PGMEA);多聚赖氨酸(PDL);4',6-二脒基-2-苯基吲哚(DAPI)染色液;RPMI培养基;青霉素-链霉素;胎牛血清;人肾透明细胞腺癌细胞(786-O细胞);罗丹明标记的鬼笔环肽;Mito-Tracker Green荧光探针;碘化丙啶(PI);NaCl、KCl、KH2PO4 和Na2HPO4;无水乙醇;超纯水。实验中所有试剂均直接使用,未作纯化处理。
本实验中所用仪器包括:台式匀胶机;飞秒激光器;无掩模光学投影光刻系统(自主搭建);等离子体表面处理仪;离子溅射仪;扫描电子显微镜(SEM);原子力显微镜(AFM);激光扫描共聚焦显微镜(LSCM);接触角仪。
2.2 拓扑结构的制备
首先将稀释的正性光刻胶AZ P4620(质量比为MAZ P4620∶MPGMEA=1∶1)以4000 r/min的速度旋涂在干净的盖玻片上,并在95 ℃的加热板上烘1 min。然后,将样品固定在MOPL的三维移动台上进行程序化曝光。曝光完毕后将样品置于稀释的AZ 400K显影液(体积比为VAZ400K∶V水=1∶4)中。由于是正胶,曝光部分在显影液中产生酸分解,未曝光部分被保留,从而形成所设计的图案化拓扑结构。
2.3 拓扑结构的形貌表征
首先将所制备的拓扑结构用去离子水冲洗干净并晾干。然后,在拓扑结构表面喷镀一层金膜,利用SEM进行形貌观察。SEM图像拍摄过程中采用的加速电压为10 keV。采用AFM表征拓扑结构的三维形貌,将获得的AFM图像在NanoScope Analysis 1.9软件中处理,可得到拓扑结构的三维形貌图像、截面轮廓图以及拓扑结构的高度分布信息。
2.4 细胞培养
本研究选取人肾透明细胞腺癌细胞(786-O)进行实验研究。786-O细胞培养在含有10%(体积分数)胎牛血清和1%(体积分数)青霉素-链霉素的RPMI培养基中,放置在37 ℃和含有5%(体积分数)CO2的培养箱中。待细胞覆盖率达到80%时,对细胞进行传代。接种细胞前,将拓扑结构在O2等离子体中处理7 min,并在PDL中浸泡过夜。
2.5 细胞延时成像
待细胞接种在拓扑结构上5 h后,将培养皿从培养箱中转移至活细胞工作站(37 ℃,CO2体积分数为5%),利用激光扫描共聚焦显微镜对细胞进行时间延时成像,每10 min拍摄一次并持续20 h。
2.6 荧光染色和成像
细胞在拓扑结构上生长24 h后,对细胞进行固定、染色和观察。首先去除培养基,用PBS润洗3次,每次3 min。然后在室温下加入4%多聚甲醛固定细胞15 min,之后用磷酸盐缓冲溶液(PBS)润洗3次。之后加入曲拉通TritonX-100渗透5 min,用PBS润洗3次。最后分别加入鬼笔环肽和DAPI染色液在黑暗中孵育细胞30 min和10 min,并用PBS润洗3次即可观察。利用LSCM对细胞进行荧光成像,细胞核的荧光采用405 nm连续激光激发,并在410~460 nm的波段内记录共聚焦荧光图像。肌动蛋白的荧光采用561 nm连续激光激发,在570~620 nm的波段内记录共聚焦荧光图像。
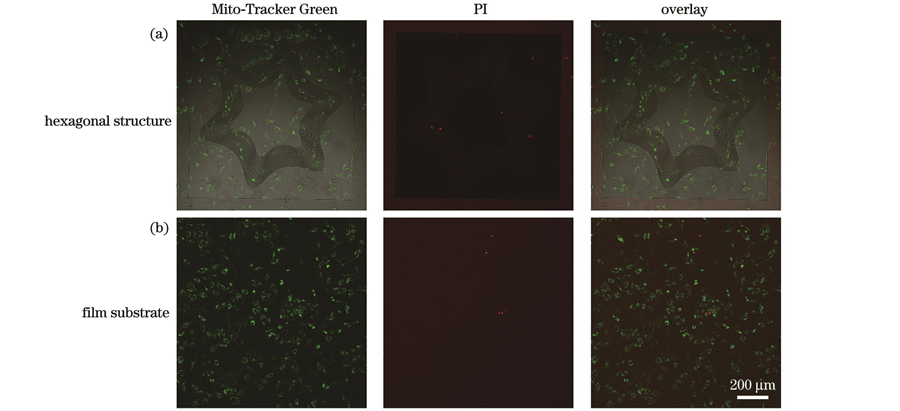
细胞活死染色步骤:细胞培养在拓扑结构上24 h后,分别加入Mito-Tracker Green和PI荧光探针孵育20 min和10 min,然后用温热的培养基润洗2次,每次3 min。利用LSCM对细胞进行成像,线粒体的荧光采用488 nm连续激光激发,并在490~540 nm波段内记录共聚焦荧光图像。细胞核的荧光采用561 nm连续激光激发,并在570~620 nm的波段内记录共聚焦荧光图像。
2.7 数据分析
微纳拓扑结构的尺寸采用ImageJ软件测量。细胞轨迹追踪采用ImageJ软件中的Manual tracking进行分析,每个样品至少有10个细胞被分析。细胞轮廓在ImageJ中手动描出,测量得出细胞铺展面积和细胞周长。通过对细胞进行椭圆拟合,得出细胞长轴与短轴的值。细胞圆度定义为短轴与长轴的比值,圆度接近1代表细胞趋于圆形,圆度接近0代表细胞拉长度大。每个样品至少有30个细胞被测量。
3 分析与讨论
3.1 拓扑结构的制备与表征
MOPL技术是一种低成本、高通量且灵活的微纳拓扑结构制备方法。在加工的过程中,MOPL中的数字微镜器件(DMD)能够根据载入的图形进行空间光场调制,从而形成相应图形的数字掩模。因此,可以通过图形设计实现所需要的微纳结构的制备。在本研究中,我们利用MOPL技术制备了一系列曲线型微纳拓扑结构,实现了细胞行为的定向调控(

图 1. 大面积微纳拓扑结构的制备及细胞行为调控示意图
Fig. 1. Schematics of fabrication of large-area micro-nano topography structures and regulation of cell behavior
考虑到正常组织所处的细胞外基质表面为弯曲形或波浪状结构,为了更有效地模拟细胞微环境,我们分别制备了折线形微纳拓扑结构和不同曲率的波浪形微纳拓扑结构来研究细胞行为,以沟槽结构作为对照。如
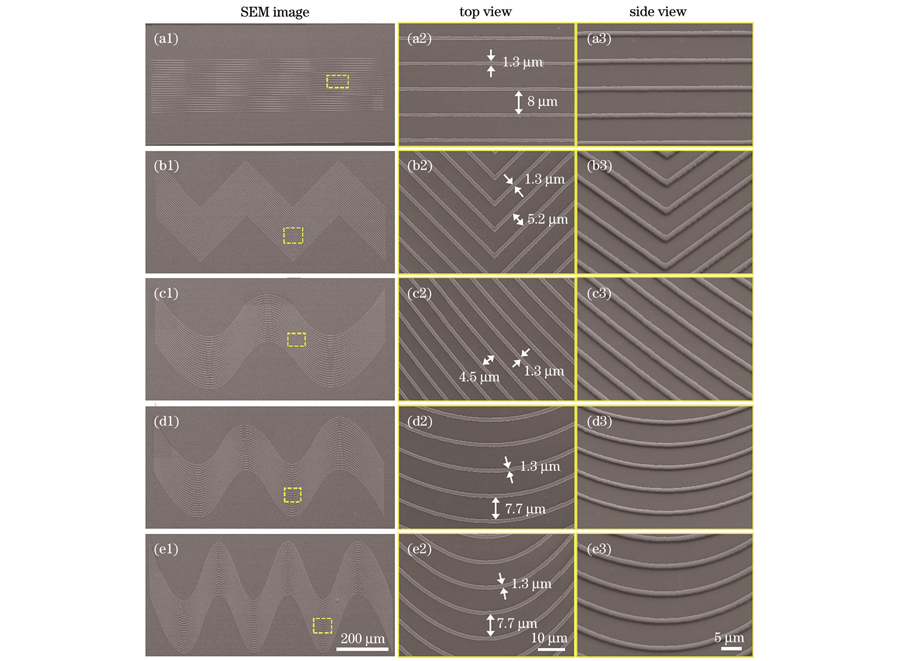
图 2. 所制备的微纳拓扑结构。(a1)~(a3)沟槽结构;(b1)~(b3)折线结构;(c1)~(c3)波浪线1结构;(d1)~(d3)波浪线2结构;(e1)~(e3)波浪线3结构
Fig. 2. Prepared micro-nano topography structures. (a1)-(a3) Groove structure; (b1)-(b3) broken line structure; (c1)-(c3) wave 1 structure; (d1)-(d3) wave 2 structure; (e1)-(e3) wave 3 structure
我们利用原子力显微镜对所制备的微纳拓扑结构的三维形貌进行了表征。如

图 3. 拓扑结构的AFM图像和接触角。(a)平面、(b)沟槽、(c)折线、(d)波浪线1、(e)波浪线2和(f)波浪线3结构的三维形貌图,下方为对应结构的截面轮廓图;(g)拓扑结构的高度统计;(h)未处理的和处理的平面基底的接触角
Fig. 3. AFM images and contact angles of topography structures. 3D morphology of (a) film, (b)groove, (c) broken line, (d) wave 1, (e) wave 2, and (f) wave 3 structures with corresponding cross-sectional profile images shown below; (g) height statistic of topography structure; (h) contact angles of untreated and treated film substrates
3.2 拓扑结构对细胞迁移行为的影响
为了研究拓扑结构对细胞迁移行为的影响,我们将786-O细胞培养在一系列所制备的微纳拓扑结构上,待细胞在结构上粘附5 h后,将细胞转移至活细胞工作站中进行延时成像观察。
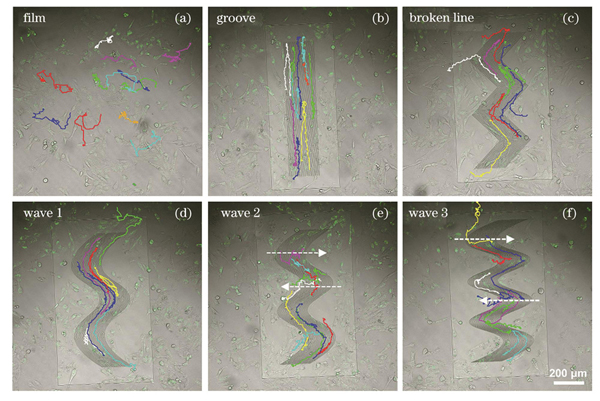
图 4. 细胞在不同结构上培养20 h的迁移轨迹,箭头指示细胞迁移方向。(a)平面;(b)沟槽;(c)折线;(d)波浪线1;(e)波浪线2;(f)波浪线3
Fig. 4. Migration trajectories of cells cultured on different structures for 20 h with directions of cell migration shown by arrows. (a) Film; (b) groove; (c) broken line; (d) wave 1; (e) wave 2; (f) wave 3
3.3 拓扑结构对细胞形态的影响与细胞行为变化机制
细胞在迁移的过程中,首先伸出细窄的丝状伪足感知外部环境,然后在迁移的方向上形成宽大的板状伪足与基底形成稳定黏附,之后细胞后方变窄并逐渐与基底脱离[45-47]。为了理解细胞在所制备的微纳拓扑结构上的迁移机制,我们利用荧光染色对拓扑结构上的细胞形态进行了可视化处理并进行了量化分析。

图 5. 拓扑结构上细胞的形态分析。(a)细胞在不同拓扑结构上的共聚焦荧光图像;(b)细胞周长统计;(c)细胞铺展面积统计;(d)细胞圆度统计;(e)细胞在不同拓扑结构上的迁移机制示意图
Fig. 5. Analysis of cell morphology on topography structures. (a) Confocal fluorescence images of cells on different topography structures; (b) statistic of cell perimeter; (c) statistic of cell spreading area; (d) statistic of cell roundness; (e) schematics of migration mechanism of cells on different topography structures
通过分析细胞在一系列拓扑结构基底上的行为,我们提出了细胞在曲线型拓扑结构上的迁移机制。如
3.4 细胞图案化调控
上述讨论表明细胞能够适应基底拓扑结构的弯曲形成相应方向的定向迁移。进一步地,我们制备了圆环形和六角形图案化结构来诱导细胞的定向迁移。
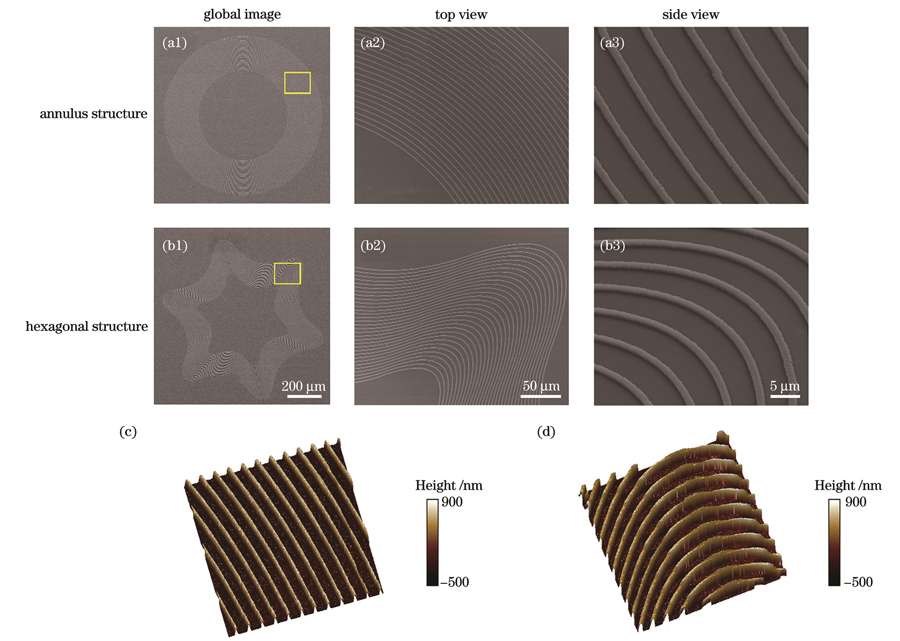
图 6. 圆环形结构和六角形结构的形貌表征。(a1)~(a3)圆环形结构的SEM图像;(b1)~(b3)六角形结构的SEM图像;(c)圆环形结构的AFM图像;(d)六角形结构的AFM图像
Fig. 6. Morphological characterization of annulus and hexagonal structures. (a1)-(a3) SEM images of annulus structures; (b1)-(b3) SEM images of hexagonal structures; (c) AFM image of annulus structure; (d) AFM image of hexagonal structure
在图案化拓扑结构上培养细胞20 h后,对其进行固定和染色观察。
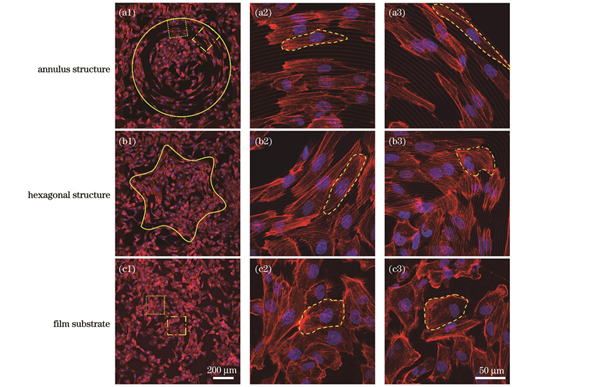
图 7. 细胞在图案化拓扑结构上的共聚焦荧光图像,虚线表示细胞轮廓。(a1)圆环形结构上细胞的荧光图像;(a2)图7(a1)点框处放大的荧光图像;(a3)图7(a1)点线框处放大的荧光图像;(b1)六角形结构上细胞的荧光图像;(b2)局部放大的线区域的荧光图像;(b3)局部放大的拐角区域的荧光图像;(c1)平面基底上细胞的荧光图像;(c2)图7(c1)中点框处放大的荧光图像;(c3)图7(c1)中点线框处放大的荧光图像
Fig. 7. Confocal fluorescence images of cells on patterned topography structures with cell outline shown by dashed line. (a1) Fluorescence image of cells on annulus structure; (a2) magnified fluorescence image at dot box in Fig. 7(a1); (a3) magnified fluorescence image at dotted wireframe in Fig. 7(a1); (b1) fluorescence image of cells on hexagonal structure; (b2) local magnification of fluorescence image in line region; (b3) local magnification of fluorescence image in corner region; (c1) fluorescence image of cell on film substrate; (c2) magnified fluorescence image at dot box in Fig. 7(c1); (c3) magnified fluorescence image at dotted wireframe in Fig. 7(c1)
3.5 拓扑结构的生物相容性
为了验证所制备的微纳拓扑结构的生物相容性,我们将细胞分别接种在拓扑结构和光刻胶薄膜上进行共培养。24 h后,分别使用Mito-Tracker Green和PI荧光探针对活细胞的线粒体和死细胞的细胞核进行染色。如
4 结论
利用飞秒激光无掩模投影光刻技术制备了系列曲线型微纳拓扑结构,并详细研究了细胞的迁移行为和细胞形态变化。结果表明:细胞能够按照一系列直线、折线、曲线拓扑结构进行生长、迁移。当波浪线结构曲率过高时,细胞改变原有的迁移方向,出现沿弯角方向的迁移行为。这是由于在波浪线结构的拐角区域,细胞骨架发生重排,因此细胞形态发生变化,进而细胞的迁移方向发生改变。因此,拓扑结构的形貌在细胞的迁移过程中发挥着重要作用。进一步地,制备了圆环形和六角形图案化拓扑结构,细胞能够严格按照图案化结构形貌进行定向迁移,从而实现细胞图案化。所制备的拓扑结构与细胞的共培养实验证实其具备良好的生物相容性。该研究表明,拓扑结构可以明显调控细胞的形态、生长和迁移行为。研究结果进一步加深了对拓扑结构调控细胞行为机制的理解。
[1] Lee D B, Kim D W, Cho J Y. Role of growth factors in hematopoietic stem cell niche[J]. Cell Biology and Toxicology, 2020, 36(2): 131-144.
[2] Zhang H L, Zheng X W, Ahmed W, et al. Design and applications of cell-selective surfaces and interfaces[J]. Biomacromolecules, 2018, 19(6): 1746-1763.
[3] Charras G, Sahai E. Physical influences of the extracellular environment on cell migration[J]. Nature Reviews Molecular Cell Biology, 2014, 15(12): 813-824.
[4] Li J, Di Russo J, Hua X M, et al. Surface immobilized E-cadherin mimetic peptide regulates the adhesion and clustering of epithelial cells[J]. Advanced Healthcare Materials, 2019, 8(8): 1801384.
[5] Damiati L A, Tsimbouri M P, Hernandez V L, et al. Materials-driven fibronectin assembly on nanoscale topography enhances mesenchymal stem cell adhesion, protecting cells from bacterial virulence factors and preventing biofilm formation[J]. Biomaterials, 2022, 280: 121263.
[6] Han L, Yin Q D, Yang L L, et al. Biointerface topography regulates phenotypic switching and cell apoptosis in vascular smooth muscle cells[J]. Biochemical and Biophysical Research Communications, 2020, 526(3): 841-847.
[7] Yang L L, Ge L, van Rijn P. Synergistic effect of cell-derived extracellular matrices and topography on osteogenesis of mesenchymal stem cells[J]. ACS Applied Materials & Interfaces, 2020, 12(23): 25591-25603.
[8] Li B, Chen J X, Wang J H C. RGD peptide-conjugated poly(dimethylsiloxane) promotes adhesion, proliferation, and collagen secretion of human fibroblasts[J]. Journal of Biomedical Materials Research Part A, 2006, 79(4): 989-998.
[9] Engler A J, Sen S, Sweeney H L, et al. Matrix elasticity directs stem cell lineage specification[J]. Cell, 2006, 126(4): 677-689.
[10] Wang S, Hashemi S, Stratton S, et al. The effect of physical cues of biomaterial scaffolds on stem cell behavior[J]. Advanced Healthcare Materials, 2021, 10(3): 2001244.
[11] Liu X L, Wang S T. Three-dimensional nano-biointerface as a new platform for guiding cell fate[J]. Chemical Society Reviews, 2014, 43(8): 2385-2401.
[12] Niari S A, Rahbarghazi R, Geranmayeh M H, et al. Biomaterials patterning regulates neural stem cells fate and behavior: the interface of biology and material science[J]. Journal of Biomedical Materials Research Part A, 2022, 110(3): 725-737.
[13] Thrivikraman G, Jagiełło A, Lai V K, et al. Cell contact guidance via sensing anisotropy of network mechanical resistance[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(29): e2024942118.
[14] Kim J, Bae W G, Kim Y J, et al. Directional matrix nanotopography with varied sizes for engineering wound healing[J]. Advanced Healthcare Materials, 2017, 6(19): 1700297.
[15] Gui N, Xu W, Myers D E, et al. The effect of ordered and partially ordered surface topography on bone cell responses: a review[J]. Biomaterials Science, 2018, 6(2): 250-264.
[16] Li G C, Li S J, Zhang L L, et al. Construction of biofunctionalized anisotropic hydrogel micropatterns and their effect on schwann cell behavior in peripheral nerve regeneration[J]. ACS Applied Materials & Interfaces, 2019, 11(41): 37397-37410.
[17] Tamiello C, Buskermolen A B C, Baaijens F P T, et al. Heading in the right direction: understanding cellular orientation responses to complex biophysical environments[J]. Cellular and Molecular Bioengineering, 2016, 9(1): 12-37.
[18] Matschegewski C, Staehlke S, Loeffler R, et al. Cell architecture-cell function dependencies on titanium arrays with regular geometry[J]. Biomaterials, 2010, 31(22): 5729-5740.
[19] Staehlke S, Koertge A, Nebe B. Intracellular calcium dynamics dependent on defined microtopographical features of titanium[J]. Biomaterials, 2015, 46: 48-57.
[20] Matschegewski C, Staehlke S, Birkholz H, et al. Automatic actin filament quantification of osteoblasts and their morphometric analysis on microtextured silicon-titanium arrays[J]. Materials, 2012, 5(7): 1176-1195.
[21] Fischer R S, Sun X Y, Baird M A, et al. Contractility, focal adhesion orientation, and stress fiber orientation drive cancer cell polarity and migration along wavy ECM substrates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(22): 2021135118.
[22] Tilbury K B, Campbell K R, Eliceiri K W, et al. Stromal alterations in ovarian cancers via wavelength dependent Second Harmonic Generation microscopy and optical scattering[J]. BMC Cancer, 2017, 17(1): 102.
[23] Provenzano P P, Eliceiri K W, Campbell J M, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion[J]. BMC Medicine, 2006, 4(1): 38.
[24] Conklin M W, Gangnon R E, Sprague B L, et al. Collagen alignment as a predictor of recurrence after ductal carcinoma in situ[J]. Cancer Epidemiology, Biomarkers & Prevention, 2018, 27(2): 138-145.
[25] Chuang Y J, Tseng F G, Lin W K. Reduction of diffraction effect of UV exposure on SU-8 negative thick photoresist by air gap elimination[J]. Microsystem Technologies, 2002, 8(4): 308-313.
[26] Park J H, Steingart D A, Kodambaka S, et al. Electrochemical electron beam lithography: write, read, and erase metallic nanocrystals on demand[J]. Science Advances, 2017, 3(7): e1700234.
[27] Córdoba R, Ibarra A, Mailly D, et al. Vertical growth of superconducting crystalline hollow nanowires by He+ focused ion beam induced deposition[J]. Nano Letters, 2018, 18(2): 1379-1386.
[28] McGehee W R, Michels T, Aksyuk V, et al. Two-dimensional imaging and modification of nanophotonic resonator modes using a focused ion beam[J]. Optica, 2017, 4(11): 1444-1450.
[29] Malloy M, Litt L C. Technology review and assessment of nanoimprint lithography for semiconductor and patterned media manufacturing[J]. Journal of Micro/Nanolithography, MEMS, and MOEMS, 2011, 10(3): 032001.
[30] Bhardwaj N, Kundu S C. Electrospinning: a fascinating fiber fabrication technique[J]. Biotechnology Advances, 2010, 28(3): 325-347.
[31] Jin F, Liu J, Zhao Y Y, et al. λ/30 inorganic features achieved by multi-photon 3D lithography[J]. Nature Communications, 2022, 13(1): 1-10.
[32] Wang J Y, Jin F, Dong X Z, et al. Flytrap inspired pH-driven 3D hydrogel actuator by femtosecond laser microfabrication[J]. Advanced Materials Technologies, 2022, 7(8): 2200276.
[33] Gao W, Chao H, Zheng Y C, et al. Ionic carbazole-based water-soluble two-photon photoinitiator and the fabrication of biocompatible 3D hydrogel scaffold[J]. ACS Applied Materials & Interfaces, 2021, 13(24): 27796-27805.
[34] 高文, 郑美玲, 金峰, 等. 飞秒激光快速制备大面积二维微纳结构[J]. 激光与光电子学进展, 2020, 57(11): 111421.
[35] 陈林森, 乔文, 叶燕, 等. 面向柔性光电子器件的微纳光制造关键技术与应用[J]. 光学学报, 2021, 41(8): 0823018.
[36] Wang T W, Dong X Z, Jin F, et al. Consistent pattern printing of the gap structure in femtosecond laser DMD projection lithography[J]. Optics Express, 2022, 30(20): 36791-36801.
[37] 匡珺洁, 罗宁宁, 张静雅, 等. 基于空间光调制器的并行微纳光刻技术研究进展[J]. 激光与光电子学进展, 2022, 59(11): 1100009.
[38] 周子逸, 董贤子, 郑美玲. 数字微镜无掩模光刻技术进展及应用[J]. 激光与光电子学进展, 2022, 59(9): 0922030.
[39] Liu Y H, Zhao Y Y, Jin F, et al. λ/12 super resolution achieved in maskless optical projection nanolithography for efficient cross-scale patterning[J]. Nano Letters, 2021, 21(9): 3915-3921.
[40] 王荣荣, 张维彩, 金峰, 等. 双光子聚合制备聚苯胺微结构[J]. 中国激光, 2021, 48(2): 0202006.
[41] 张维彩, 郑美玲, 董贤子, 等. 高精细水凝胶微图案的快速制备及其对细胞行为的诱导[J]. 光电工程, 2022, 49(2): 0210336.
Zhang W C, Zheng M L, Dong X Z, et al. Rapid preparation of high-precision hydrogel micropatterns and its induction of cell behavior[J]. Opto-Electronic Engineering, 2022, 49(2): 0210336.
[42] Kang M S, Han C, Jeon H. Submicrometer-scale pattern generation via maskless digital photolithography[J]. Optica, 2020, 7(12): 1788-1795.
[43] Jung Y, Lee H, Park T J, et al. Programmable gradational micropatterning of functional materials using maskless lithography controlling absorption[J]. Scientific Reports, 2015, 5(1): 1-7.
[44] Shi W X, Xu T L, Xu L P, et al. Cell micropatterns based on silicone-oil-modified slippery surfaces[J]. Nanoscale, 2016, 8(44): 18612-18615.
[45] Ridley A J, Schwartz M A, Burridge K, et al. Cell migration: integrating signals from front to back[J]. Science, 2003, 302(5651): 1704-1709.
[46] Pollard T D, Borisy G G. Cellular motility driven by assembly and disassembly of actin filaments[J]. Cell, 2003, 112(4): 453-465.
[47] Ladoux B, Nicolas A. Physically based principles of cell adhesion mechanosensitivity in tissues[J]. Reports on Progress in Physics, 2012, 75(11): 116601.
Article Outline
郭敏, 刘享洋, 董贤子, 刘洁, 金峰, 郑美玲. 曲线型微纳拓扑结构的制备及细胞行为调控[J]. 中国激光, 2023, 50(15): 1507303. Min Guo, Xiangyang Liu, Xianzi Dong, Jie Liu, Feng Jin, Meiling Zheng. Fabrication of Curved Micro-Nano Topography Structures and Regulation of Cell Behavior[J]. Chinese Journal of Lasers, 2023, 50(15): 1507303.