Wide-field ophthalmic space-division multiplexing optical coherence tomography  Download: 526次
Download: 526次
1. INTRODUCTION
Since its inception in 1991, optical coherence tomography (OCT) has revolutionized diagnostic care for retinal diseases [1,2]. The ability to resolve high-resolution cross-sectional and volumetric images of the macula has been key for diagnosis of retina diseases like age-related macular degeneration, macular edema, diabetic retinopathy, and retinitis pigmentosa [3
One of the key image quality problems in ophthalmic OCT is motion artifacts [10,11]. Many ophthalmic patients are elderly and have the inability to sit still for more than a few seconds. Additional sources of motion artifacts include involuntary micro saccades and even the patient’s breaths and heartbeats. To reduce the probability of motion artifacts, ophthalmic OCT images must be acquired rapidly, or motion tracking hardware and software must be employed [12]. Typical OCT eye-tracking systems employ a scanning laser ophthalmoscope (SLO) to produce en face images of the retina that the OCT scans can be registered against [13]. This increases system complexity and may not have high enough registration performance to acquire accurate volumetric scans [14]. Most commercial OCT systems are limited by scan speeds of tens of kilohertz. Due to the large number of A-scans required and extended scan periods, OCT volume scans with commercial systems are often untenable, even over small scan ranges. Many commercial systems use a range of scan patterns, including tens of B-scans over small scan ranges (up to
Because this is an important problem, the development of high-speed OCT systems has been an active area of research [14–
Space-division multiplexing optical coherence tomography (SDM-OCT) is a high-speed OCT system that takes advantage of the long coherence length of VCSELs to multiplex multiple images along the imaging depth [22]. This parallel imaging technique splits the sample arm light into multiple channels and imparts an optical path length delay to each channel. Each of these imaging channels illuminates a different position on the sample, enabling simultaneous imaging of multiple locations, increasing the effective image speed. VCSELs can have coherence lengths on the order of centimeters, whereas the penetration depth of near-infrared (NIR) OCT imaging light is typically less than 2 mm [23]. This mismatch between laser coherence length and imaging penetration depth means that the total amount of information that the interference fringe can carry is not being fully utilized with the use of a single imaging beam. By adding optical path length delays longer than the optical penetration depth in tissue to the parallel channels, parallel images can be acquired simultaneously without overlapping. The advantage of SDM-OCT is that the multiple imaging beams can be acquired by a single detector, which has reduced system complexity compared to other multi-beam OCT systems [24
Table 1. Comparison of SDM-OCT Systems
|
2. METHODS
2.1 A. System Description
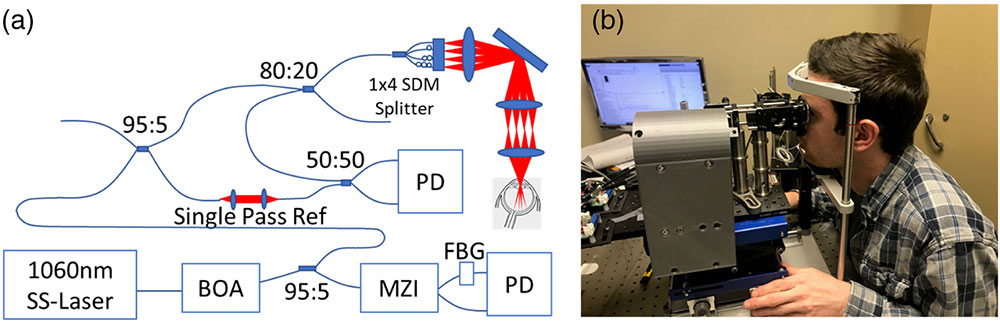
The system source is a 1060 nm MEMs VCSEL swept-source operating at 200 kHz. This source has an output power of 35 mW and a (a) Schematic for the ophthalmic SDM-OCT system. BOA, booster optical amplifier; FBG, fiber Bragg grating; MZI, Mach–Zehnder interferometer; PD, balanced photodetector. (b) Picture of the ophthalmic SDM-OCT prototype in the clinic.
1. Safety Considerations
The ophthalmic SDM-OCT system was approved for human imaging by institutional review boards (IRBs) at both Lehigh University and University of Pennsylvania. The ANSI standard prescribes the maximum power incident on the eye for a 1060 nm single laser source as 1.8 mW [32]. This system utilizes
2. Sample Arm Optics Design
Choosing an appropriate interbeam optical path distance is important because we must consider the penetration depth of a single beam, the curvature of the eye, and the system imaging range. Also, the lateral displacement of the imaging beams is important to satisfy the ANSI standard for distributed sources. The fiber tips are laterally spaced by 500 μm on the custom
The theoretical sensitivity of a single-beam OCT system operating at 200 kHz with a 68% duty cycle, 1.8 mW power on the sample, and 0.7 detector sensitivity is
2.2 B. Image Processing
SDM-OCT fringes were acquired in parallel with MZI calibration fringes. Every SDM-OCT A-scan fringe was registered to the FBG peak and interpolated based on the MZI calibration fringe. SDM-OCT frames were discrete Fourier transform registered to reduce axial motion artifacts [34]. The four SDM-OCT beams were manually stitched using ImageJ. A mean filter was applied to reduce speckle noise. Stitched SDM-OCT images were flattened by segmenting the retinal pigment epithelium (RPE) with a graph cut segmentation technique [35]. Cross-sectional images and en face projections were extracted from the flattened image. Retina thickness maps were generated by segmentation of internal limiting membrane (ILM) and the RPE. The axial positions of the ILM and RPE were subtracted for each frame to calculate the two-dimensional (2D) retinal thickness. For visualization, retinal thickness was mapped to hue and projected onto an en face projection of the RPE.
2.3 C. Clinical Feasibility Study
A clinical feasibility study was conducted at Scheie Eye Institute at the University of Pennsylvania. Ten patients between the ages of 18 and 80 with previous diagnosis of retinal disease were recruited. SDM-OCT images were acquired for both of each patient’s eyes. Reference OCT images were acquired with commercial OCT systems (Heidelberg Spectralis or Zeiss Cirrus).
3. RESULTS
3.1 A. System Characterization

Fig. 2. Geometric considerations for ophthalmic SDM-OCT design. (a) Projection of the curvature of the eye onto the imaging space. (b) Image space separated into four imaging beams with 3 mm optical delay. All four beams fit in the 12 mm image depth. (c) Distance between adjacent beams shows there is no overlapping between adjacent images. (d) Sensitivity roll-off measured over the entire imaging depth range.
Figure
3.2 B. Tests on Healthy Subjects

Fig. 3. (a) Four-beam raw SDM-OCT image. (b) En face projection of the RPE layer; yellow line shows location of vertical cross section and green line shows location of horizontal cross-section. (c) Vertical cross-section. (d) Horizontal cross-section with some selected anatomical features labeled. CHR, choroid; EZ, ellipsoid zone; ILM, internal limiting membrane; IPL, inner plexiform layer; ONH, optic nerve head; OPL, outer plexiform layer; RPE, retinal pigment epithelium. (e) Retinal thickness map. (f) 3D rendering of stitched SDM-OCT images (also see Visualization 1 ). Scale bars 1 mm.
3.3 C. Clinical Feasibility Study

Fig. 4. SDM-OCT and commercial OCT images from a patient diagnosed with retinal telangiectasia. (a) En face projection SDM-OCT. (b) Vertical cross-section SDM-OCT. (c) Horizontal cross-section SDM-OCT. (d) En face SLO image of commercial system imaging range (Visualization 2 ). Lateral scale bars 1 mm. Axial scale bars 500 μm for (b), (c), (e), and (f) and 200 μm for (g) and (h).

Fig. 5. SDM-OCT and commercial OCT images from a patient diagnosed with exudative age-related macular degeneration. (a) En face projection of RPE SDM-OCT. (b) Vertical cross-section SDM-OCT. (c) Horizontal cross-section SDM-OCT. (d) En face SLO image of commercial system imaging range. (e) Vertical cross-section commercial OCT. (f) Horizontal cross-section commercial OCT. (g) Zoomed-in ROI from SDM-OCT. (h) Zoomed-in ROI from commercial OCT. (i) Retinal thickness map. Yellow arrow indicates region with retinal thinning. (j) 3D rendering of stitched SDM-OCT images (also see Visualization 3 ). Lateral scale bars 1 mm. Axial scale bars 500 μm for (b), (c), (e), and (f) and 200 μm for (g) and (h).
Another patient was diagnosed with non-proliferative diabetic retinopathy in his left eye. A similar comparison between SDM-OCT and commercial OCT images is made in Fig.
4. DISCUSSION
The SDM-OCT system we demonstrated here utilizes a parallel imaging approach to acquire volumetric retinal images at speeds
The clinical feasibility study conducted here showed good matching between pathological features seen in SDM-OCT images and commercial OCT images. We were able to acquire wide-field structural images in less than 1 s. These wide-field volumetric images had high resolution over an imaging range of
Although SDM-OCT offers key advantages in imaging speed, there are some limitations. The biggest drawback of this method at this stage is the relatively low imaging sensitivity, which results in more-noisy images and requires extensive filtering to improve image contrast. This system has a sensitivity of 91 dB, whereas commercial OCT systems typically operate with sensitivities greater than 100 dB. This low sensitivity is principally caused by the 6 dB backpropagation loss through the
One another limitation of this method is the limited flexibility of the scan patterns that can be used with this method. The scan range along the vertical direction is fixed due to the fixed separation distance of the parallel imaging beams. Changing the distance between beams is possible with changes to sample arm optics, but the minimum distance between beams is limited by the ANSI definition of distributed sources. Beams that are closer than this limit increase the risk of thermal injury to the retina. This limits the ability to perform small-range, high-resolution, high-density scan patterns that are comparable to commercial OCT macula scan patterns. Additional limitations include the beam power nonuniformity, which can cause differences in image brightness between imaging beams and the necessity to stitch the four parallel images together, which can be a time-consuming process if done manually.
5. CONCLUSION
We demonstrate an ophthalmic SDM-OCT system, which can acquire retinal depth sections at an equivalent A-scan rate of 800 kHz. This allows rapid acquisition of volumetric, wide-field retina images for the diagnosis of retina diseases. A clinical feasibility study was conducted to analyze the image quality of the ophthalmic SDM-OCT prototype and make comparisons between SDM-OCT images and commercial OCT images. These high-speed, wide-field SDM-OCT acquisitions may reduce motion artifacts and prevent missed diagnosis of peripheral retinal disease.
6 Acknowledgment
Acknowledgment. The authors would like to thank Mrs. Joan Dupont and Dr. Benjamin Kim for their assistance in the recruitment of patients in this study.
Article Outline
Jason Jerwick, Yongyang Huang, Zhao Dong, Adrienne Slaudades, Alexander J. Brucker, Chao Zhou. Wide-field ophthalmic space-division multiplexing optical coherence tomography[J]. Photonics Research, 2020, 8(4): 04000539.